Water Basics

Despite its complex role in life and the ecosystem, water’s chemical makeup is relatively straightforward: H2O. Its structure is simple, symmetric, and nonlinear.
Water has a strong dipole moment, which makes it a polar molecule. The charge is unequally distributed such that it “points” in a particular direction. Polarity has several important consequences. For example, the polarity causes airborne water vapor to absorb infrared radiation, making it the atmosphere’s most important naturally occurring greenhouse gas. Water’s contribution to atmospheric warming creates Earth’s comfortable climate. Without water vapor and preindustrial carbon dioxide in the atmosphere, and without their propensity to absorb radiation, Earth’s atmosphere would be about 35°C cooler. Water vapor bears responsibility for approximately 20 of those degrees.
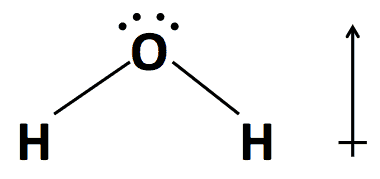
Water has a simple, symmetric structure with a strong dipole moment, which makes it a valuable solvent and the atmosphere’s most important greenhouse gas.
Water’s polarity also makes it a valuable universal solvent. It can dissolve materials, including salts, sugars, and acids. Its strong dipole moment gives water a quality called adhesion, which is a “stickiness” to other surfaces as well as other water molecules. The net effect of adhesion is water’s high surface tension, a feature that offers many benefits to living things that need to circulate water through their systems.

Water also has a relatively high heat capacity and heat of vaporization. These attributes mean that water can absorb a lot of heat before it boils. Because of its heat capacity, water makes an excellent coolant for power plants. Because water’s heat capacity is about four times higher than air, power plants would require four times as much air movement to accomplish the same level of cooling as water.
Water can exist in three phases in nature simultaneously: solid icebergs floating on a liquid ocean below a sky with clouds of water vapor. The water reaches maximum density at 4°C, meaning that ice floats on water, which is an unusual characteristic. If ice sank like most other solids, lakes would fill with ice in winter as ice sank to the bottom. Instead, the ice forms at the top, hovering above a large body of liquid teeming with life. If ice sank, dense blocks of ice would push living creatures from bodies of water, depriving them of a chance to thrive. Solid water’s lower density allows physical changes in water to occur (freezing and thawing) while life continues below.
Image Credits: Olga Nikonova/Shutterstock.com.


