Water in Fuels

In rough orders of magnitude, refining gasoline from conventional petroleum requires a few gallons of water per gallon of gasoline, irrigated corn consumes 1,000 gallons of water per gallon of ethanol, and creating biodiesel from algae uses 100,000 gallons of water per gallon of biodiesel. 1 K. M. Twomey, C. M. Beal, C. W. King, and M. E. Webber, “Biofuels: An energy and water conundrum,” World Energy Monitor 3 (2012); and C. W. King and M. E. Webber, “Water Intensity of Transportation,” Environmental Science and Technology 42 (2008), 7866-7872, doi: 10.1021/es800367m. However, a gallon of ethanol has roughly two-thirds the energy of a gallon of gasoline. Separately, the energy density per gallon of natural gas and hydrogen varies at different pressures and temperatures. Complicating matters, electricity cannot be measured by volume, which makes such comparisons of the water intensity of fuels difficult to assess.
Direct comparisons, such as gallons of water per unit of energy, are more precise than the volume-to-volume approach since the energy density variations from gasoline to ethanol, natural gas and hydrogen can more easily be accommodated. And, the energy content of electricity can also be incorporated, despite having a non obvious volume.
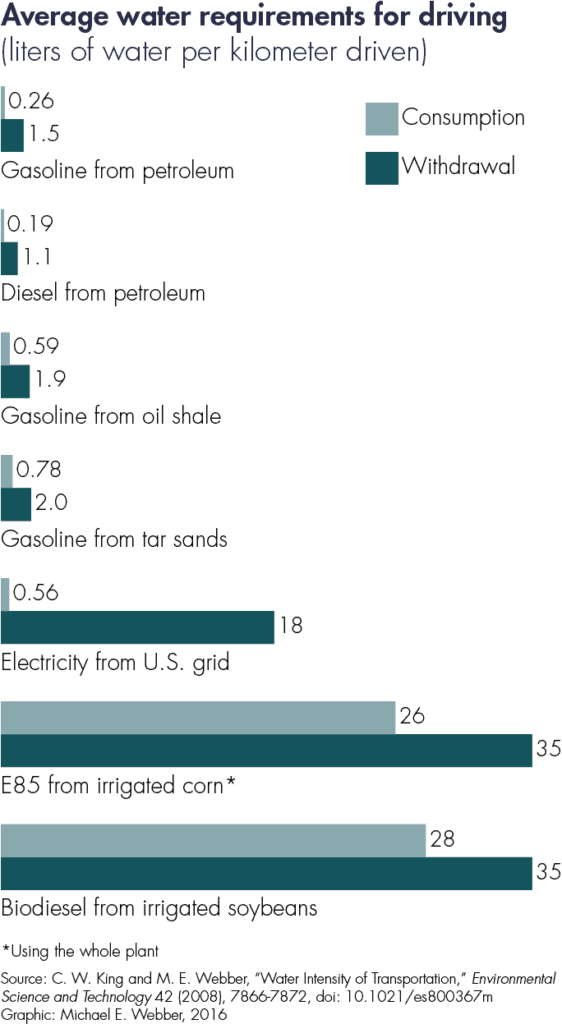
However, the comparison by energetic density excludes variations in conversion efficiency for the different fuels in the power trains. An internal combustion engine has a typical efficiency range of 15% to 30%. Motors that propel electric cars have a typical efficiency of 80% to 90%. Therefore, even the water-per-unit-of-energy metric fails to capture the fact that a unit of electrical energy takes a car three times farther than a similar amount of energy in the form of gasoline.
Taking the energy density of the fuel and the efficiency of the drivetrain together indicates a potentially more useful metric of water consumed per mile traveled. 2 C. W. King and M. E. Webber, “Water Intensity of Transportation,” Environmental Science and Technology 42 (2008), 7866-7872, doi: 10.1021/es800367m. Switching parlance from miles per gallon (of gasoline) to gallons (of water) consumed per mile traveled could be a clearer and more consistent way to compare the water intensity of transportation fuels.
Beyond these simple ratios, water is used at various stages of the life cycle of fuel production and consumption. These stages include production, upgrade, transport, and end use. Production is the point of extraction for oil and gas, mining for coal and uranium, or photosynthesis for biofuels. Upgrading includes refining crude oil into gasoline, jet fuel, and diesel; biorefining corn or sugar into ethanol; and enrichment of ore or yellowcake into uranium fuels. For oil and gas, water is used to extract the fuels from the ground by flooding conventional reservoirs, through steam-assisted gravity drainage (SAGD) for the Canadian tar sands, or by hydraulic fracturing of shale formations. For coal mining, dewatering a mine precedes coal removal. Water also helps control dust at coal mines. For uranium, water helps separate some desired minerals and also cools the power plants that provide the electricity for centrifugal enrichment. Refineries use more water both as an input chemical and to make steam. For biocrops, irrigation and steam for fermentation consume water.