Entropy: The Second Law of Thermodynamics

The second law of thermodynamics describes changes to entropy (or disorder) for a system. The law arises from the empirical observations of increasing disorder and the conclusion that processes have a direction. For example, leaves move from an ordered state (neatly attached to the tree) to a disordered state (scattered about the ground). Many have seen leaves falling, but none have witnessed fallen leaves reattaching themselves to the tree.

Similarly, heat flows from a higher to a lower temperature. For example, a cup of hot herbal tea cools when left alone in a house at room temperature as heat from the tea flows to the room around it. The tea cools while the room warms very slightly.
The second law of thermodynamics manifests as inefficiencies, losses, and waste streams during energy conversion, such as waste heat, lost fuel, or suboptimal operation of systems. Inefficiencies are simultaneously a vexing problem and an enticing opportunity for the global energy system. The United States consumes about 105 Exajoules (EJ) (about 100 quads or quadrillion British thermal units (Btu)) of energy each year. More than half of that energy—about 62 EJ (59 quads)—enters the atmosphere as waste heat from smokestacks and tailpipes or the hydrosphere as warmed water from power plants. Other waste streams include food waste, municipal solid waste, agricultural waste (manure), wastewater, and flue gases. Successfully harnessing wasted energy would be a valuable step for solving global energy problems.
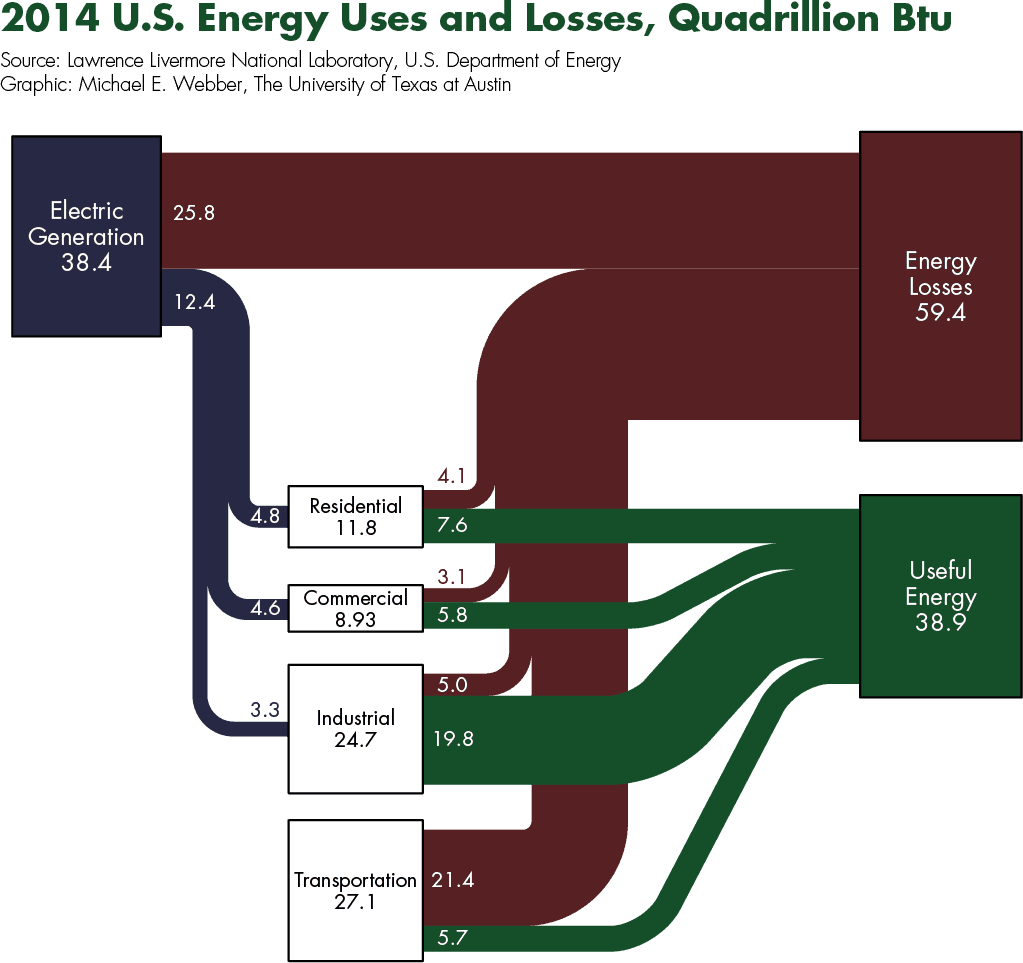
We can measure efficiency by comparing the energy output of a system to energy inputs. Highly efficient systems convert more than 90% of the incoming energy into useful output energy, though in a different form. Some anthropogenic processes, such as electricity generators and boilers, are very efficient, while others such as steam turbines and incandescent lightbulbs are not. Many resilient and robust natural processes are not very efficient. For example, photosynthesis converts on average less than 1% of energy in photons from the sun into chemical energy stored in a plant’s biomaterial.